ARE YOU A HEALTHCARE PROFESSIONAL?
This site is intended for US healthcare professionals only.
By clicking YES, you attest that you are a healthcare professional licensed in the US.
Helping patients get access to their prescribed medications with the information and support they need.
IPSEN CARESTM (Coverage, Access, Reimbursement, and Education Support) serves as a central point of contact between patients/caregivers, healthcare providers (HCPs), insurance companies, and specialty pharmacies.
*For eligibility requirements, please see terms and conditions below, or visit www.IpsenCares.com.
Patient Assistance
For patients with spasticity, Dysport has national coverage without restrictions for:

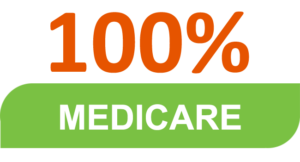
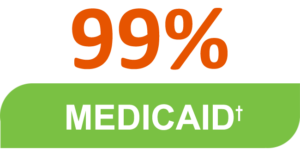
For adult patients with cervical dystonia, Dysport has national coverage without restrictions for:



*Data provided by Breakaway Partners Essentials and current as of February 2021
+Includes Managed and State Medicaid
952-846-7942
www.childneurologyfoundation.org
1-800-377-3978
dystonia-foundation.org
1-800-487-8385
torticollis.org
C.L.I.M.B. is a learning continuum designed to help physicians new to and experienced with botulinum toxin therapy improve their clinical skills involving the appropriate use of Dysport.
Learn more at climb-training.com
Click here to visit the Dysport Education page for additional training resources and information.
General Resources
 Adventure Book
Adventure BookAn interactive book for kids with spasticity to discuss their goals and accompany them throughout their treatment journey
Indication-Specific Resources
*Patient Eligibility & Terms and Conditions: Patients are not eligible for copay assistance through IPSEN CARES if they are enrolled in any state or federally funded programs for which drug prescriptions or coverage could be paid in part or in full, including, but not limited to, Medicare Part B, Medicare Part D, Medicaid, Medigap, VA, DoD, or TRICARE (collectively, “Government Programs”), or where prohibited by law. Patients residing in Massachusetts, Minnesota, Michigan, or Rhode Island can only receive assistance with the cost of Ipsen products but not the cost of related medical services (injection). Patients receiving assistance through another assistance program or foundation, free trial, or other similar offer or program, are not eligible for the copay assistance program during the current enrollment year.Cash-pay patients are eligible to participate.
“Cash-pay” patients are defined for purposes of this program as patients without insurance coverage or who have commercial insurance that does not cover Dysport. Medicare Part D enrollees who are in the prescription drug coverage gap (the “donut hole”) are not considered cash-pay patients and are not eligible for copay assistance through IPSEN CARES. For patients with commercial insurance who are not considered to be cash-pay patients, the maximum copay benefit amount per prescription is an amount equal to the difference between the annual maximum copay benefit of $5,000 and the total amount of copay benefit provided to the patient in the Dysport Copay Program. In any calendar year commencing January 1, the maximum copay benefit amount paid by Ipsen Biopharmaceuticals, Inc., will be $5,000, covering no more than four (4) Dysport treatments. For cash-pay patients, the maximum copay benefit amount per eligible Dysport treatment is $1,250, subject to the annual maximum of $5,000 in total. There could be additional financial responsibility depending on the patient’s insurance plan.
Patient or guardian is responsible for reporting receipt of copay savings benefit to any insurer, health plan, or other third party who pays for or reimburses any part of the prescription filled through the program, as may be required. Additionally, patients may not submit any benefit provided by this program for reimbursement through a Flexible Spending Account, Health Savings Account, or Health Reimbursement Account. Ipsen reserves the right to rescind, revoke, or amend these offers without notice at any time. Ipsen and/or RxCrossroads by McKesson are not responsible for any transactions processed under this program where Medicaid, Medicare, or Medigap payment in part or full has been applied. Data related to patient participation may be collected, analyzed, and shared with Ipsen for market research and other purposes related to assessing the program. Data shared with Ipsen will be de-identified, meaning it will not identify the patient. Void outside of the United States and its territories or where prohibited by law, taxed, or restricted. This program is not health insurance. No other purchase is necessary.
Postmarketing reports indicate that the effects of Dysport and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity, but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses and in approved indications, cases of spread of effect have been reported at doses comparable to or lower than the maximum recommended total dose.
Dysport is contraindicated in patients with known hypersensitivity to any botulinum toxin products, cow’s milk protein, components in the formulation or infection at the injection site(s). Serious hypersensitivity reactions including anaphylaxis, serum sickness, urticaria, soft tissue edema, and dyspnea have been reported. If such a reaction occurs, discontinue Dysport and institute appropriate medical therapy immediately.
Lack of Interchangeability Between Botulinum Toxin Products
The potency Units of Dysport are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products, and, therefore, units of biological activity of Dysport cannot be compared to or converted into units of any other botulinum toxin products assessed with any other specific assay method.
Dysphagia and Breathing Difficulties
Treatment with Dysport and other botulinum toxin products can result in swallowing or breathing difficulties. Patients with pre-existing swallowing or breathing difficulties may be more susceptible to these complications. In most cases, this is a consequence of weakening of muscles in the area of injection that are involved in breathing or swallowing. When distant side effects occur, additional respiratory muscles may be involved. Deaths as a complication of severe dysphagia have been reported after treatment with botulinum toxin. Dysphagia may persist for several weeks, and require use of a feeding tube to maintain adequate nutrition and hydration. Aspiration may result from severe dysphagia and is a particular risk when treating patients in whom swallowing or respiratory function is already compromised. Patients treated with botulinum toxin may require immediate medical attention should they develop problems with swallowing, speech, or respiratory disorders. These reactions can occur within hours to weeks after injection with botulinum toxin.
Pre-existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junction disorders (e.g., myasthenia gravis or Lambert-Eaton syndrome) should be monitored particularly closely when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including severe dysphagia and respiratory compromise from typical doses of Dysport.
Human Albumin and Transmission of Viral Diseases
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases and variant Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk of transmission would also be considered extremely remote. No cases of transmission of viral diseases, CJD, or vCJD have ever been identified for licensed albumin or albumin contained in other licensed products.
Intradermal Immune Reaction
The possibility of an immune reaction when injected intradermally is unknown. The safety of Dysport for the treatment of hyperhidrosis has not been established. Dysport is approved only for intramuscular injection.
Adults with lower limb spasticity (≥5%): falls, muscular weakness, and pain in extremity and with upper limb spasticity (≥4%): muscular weakness.
Pediatric patients with lower limb spasticity (≥10%): nasopharyngitis, cough and pyrexia and with upper limb spasticity (≥10%): upper respiratory tract infection and pharyngitis.
Adults with cervical dystonia (≥5%): muscular weakness, dysphagia, dry mouth, injection site discomfort, fatigue, headache, musculoskeletal pain, dysphonia, injection site pain, and eye disorders.
Co-administration of Dysport and aminoglycosides or other agents interfering with neuromuscular transmission (e.g., curare-like agents), or muscle relaxants, should be observed closely because the effect of botulinum toxin may be potentiated. Use of anticholinergic drugs after administration of Dysport may potentiate systemic anticholinergic effects, such as blurred vision. The effect of administering different botulinum neurotoxins at the same time or within several months of each other is unknown. Excessive weakness may be exacerbated by another administration of botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin. Excessive weakness may also be exaggerated by administration of a muscle relaxant before or after administration of Dysport.
There are no adequate and well-controlled studies in pregnant women. Dysport should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Based on animal data, Dysport may cause fetal harm.
The safety and effectiveness of Dysport injected into proximal muscles of the lower limb for the treatment of spasticity in pediatric patients has not been established. Based on animal data Dysport may cause atrophy of injected and adjacent muscles; decreased bone growth, length, and mineral content; delayed sexual maturation; and decreased fertility.
In general, elderly patients should be observed to evaluate their tolerability of Dysport, due to the greater frequency of concomitant disease and other drug therapy. Subjects aged 65 years and over who were treated with Dysport for lower limb spasticity reported a greater percentage of fall and asthenia as compared to those younger (10% vs. 6% and 4% vs. 2%, respectively).
To report SUSPECTED ADVERSE REACTIONS or product complaints, contact Ipsen at 1-855-463-5127. You may also report SUSPECTED ADVERSE REACTIONS to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Dysport® (abobotulinumtoxinA) for injection is indicated for the treatment of:
Please see full Prescribing Information, including Boxed Warning and Medication Guide.