ARE YOU A HEALTHCARE PROFESSIONAL?
This site is intended for US healthcare professionals only.
By clicking YES, you attest that you are a healthcare professional licensed in the US.
An easy ordering process with several options
Each box contains 1 sterile, single-use vial with accompanying Full Prescribing Information and Medication Guide.
If you practice within an institution please acquire Dysport from your wholesalers
For Private Practice/Clinic, click HERE
Note: For billing purposes, the NDC number requires 11 digits. Therefore, a zero must be entered into the 10th position (eg, “15054-0500-01”). This is consistent with Red Book and First Databank listings.
Ways to order
Resource Guide
The best way to ensure your patients are receiving authentic Dysport is to order from an authorized Dysport distributor. See below for the various things you can check on each Dysport package to make sure it is authentic. There are more anti-counterfeiting measures on the packaging that aren’t listed here. Ask your distributor for more information.
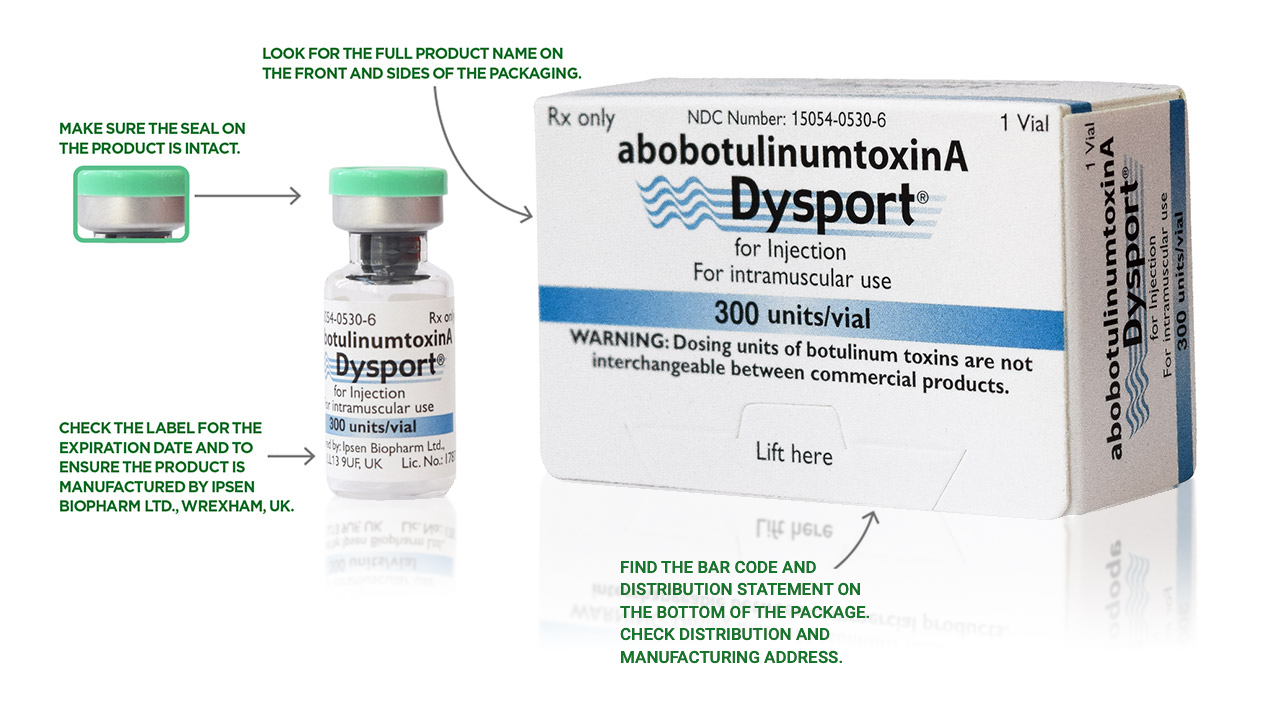
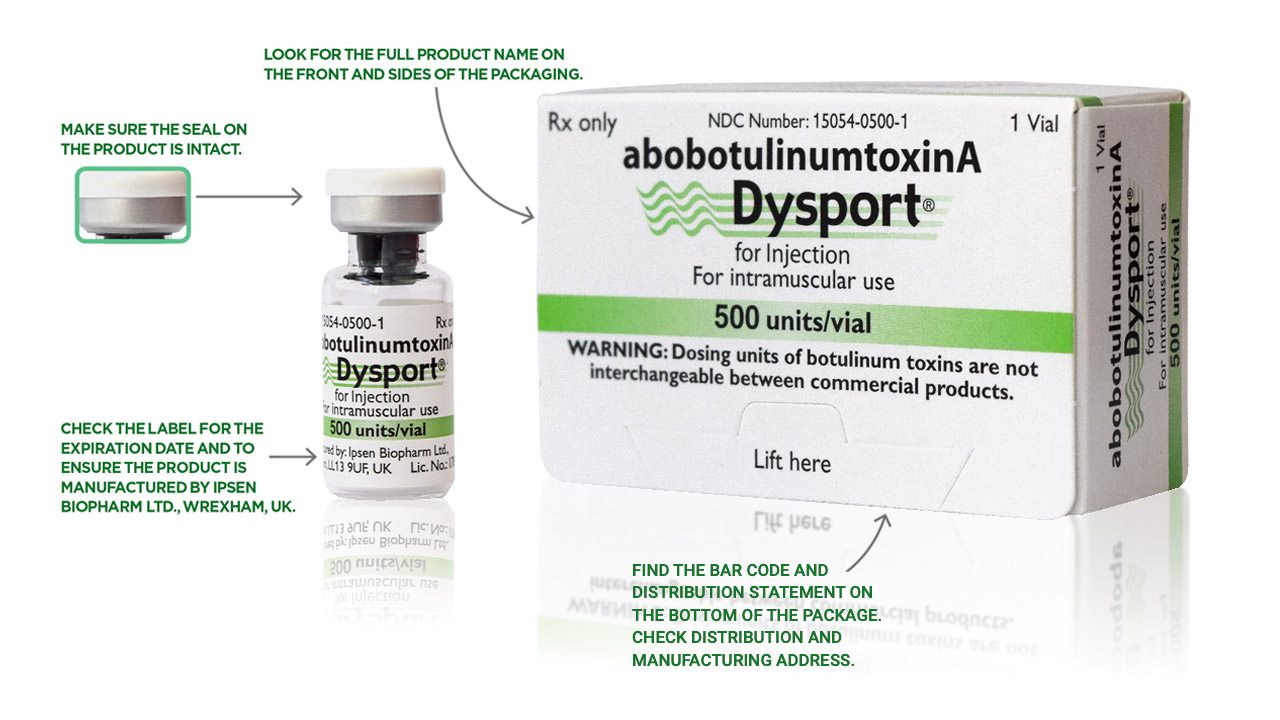
How to identify

IPSEN CARESTM Provides Support for Patients and Providers
The IPSEN CARES Patient Access Specialists are fully dedicated to:
IPSEN CARES provides a single point-of-contact dedicated to assisting patients, providers, and staff.
Phone: 1-866-435-5677
Fax: 1-866-525-2416
Hours: Monday–Friday 8:00 am–8:00 pm ET
Website: www.ipsencares.com
Ipsen realizes that more work is now being done by computer rather than paper and fax machines.We hope this online portal will be a convenient resource for you and your office. After you register and create a profile, your profile will be validated within 1 business day.
Through the online portal you can:
Visit www.ipsencares.com/hcp-resources to learn more.
 Dysport Copay Assistance Program
Dysport Copay Assistance ProgramAssistance with Private Insurance Copay or Coinsurance Costs for Dysport
EASE PATIENTS’ OUT-OF-POCKET COSTS FOR DYSPORT
SIMPLE STEPS FOR PATIENTS TO RECEIVE THEIR DYSPORT ASSISTANCE
 Patient Authorization Form
Patient Authorization FormOnce a patient is enrolled in IPSEN CARES, a Patient Authorization Form needs to be completed by the Patient/Legal Guardian every 3 years* in order to maintain participation in IPSEN CARES. The form needs to be printed, filled out completely by the Patient/Legal Guardian, signed, and faxed back to IPSEN CARES. It is important that the Patient/Legal Guardian review the original IPSEN CARES Enrollment Form prior to signing the Authorization Form.
*NOTE: The patient authorization will expire sooner than 3 years where required by state law.
 Patient Assistance Program (PAP) Application
Patient Assistance Program (PAP) ApplicationThe Patient Assistance Program (PAP) is designed to provide Dysport at no cost to eligible patients. Patients may be eligible to receive free drug if they are experiencing financial hardship, are uninsured or functionally uninsured, are a U.S. resident, and received a prescription for a use indicated in the approved label of Dysport, as supported by information provided in the program application. Eligibility does not guarantee approval for participation in the program. The PAP provides Dysport product only, and does not cover the cost of previously purchased product or medical services.
*Patient Eligibility & Terms and Conditions: Patients are not eligible for copay assistance through IPSEN CARES if they are enrolled in any state or federally funded programs for which drug prescriptions or coverage could be paid in part or in full, including, but not limited to, Medicare Part B, Medicare Part D, Medicaid, Medigap, VA, DoD, or TRICARE (collectively, “Government Programs”), or where prohibited by law. Patients residing in Massachusetts, Minnesota, Michigan, or Rhode Island can only receive assistance with the cost of Ipsen products but not the cost of related medical services (injection). Patients receiving assistance through another assistance program or foundation, free trial, or other similar offer or program, are not eligible for the copay assistance program during the current enrollment year.
Cash-pay patients are eligible to participate. “Cash-pay” patients are defined for purposes of this program as patients without insurance coverage or who have commercial insurance that does not cover Dysport. Medicare Part D enrollees who are in the prescription drug coverage gap (the “donut hole”) are not considered cash-pay patients and are not eligible for copay assistance through IPSEN CARES. For patients with commercial insurance who are not considered to be cash-pay patients, the maximum copay benefit amount per prescription is an amount equal to the difference between the annual maximum copay benefit of $5,000 and the total amount of copay benefit provided to the patient in the Dysport Copay Program. In any calendar year commencing January 1, the maximum copay benefit amount paid by Ipsen Biopharmaceuticals, Inc. will be $5,000, covering no more than four (4) Dysport treatments. For cash-pay patients, the maximum copay benefit amount per eligible Dysport treatment is $1,250, subject to the annual maximum of $5,000 in total. There could be additional financial responsibility depending on the patient’s insurance plan.
Patient or guardian is responsible for reporting receipt of copay savings benefit to any insurer, health plan, or other third party who pays for or reimburses any part of the prescription filled through the program, as may be required. Additionally, patients may not submit any benefit provided by this program for reimbursement through a Flexible Spending Account, Health Savings Account, or Health Reimbursement Account. Ipsen reserves the right to rescind, revoke, or amend these offers without notice at any time. Ipsen and/or RxCrossroads by McKesson are not responsible for any transactions processed under this program where Medicaid, Medicare, or Medigap payment in part or full has been applied. Data related to patient participation may be collected, analyzed, and shared with Ipsen for market research and other purposes related to assessing the program. Data shared with Ipsen will be de-identified, meaning it will not identify the patient. Void outside of the United States and its territories or where prohibited by law, taxed, or restricted. This program is not health insurance. No other purchase is necessary.
**Patients may be eligible to receive free drug if they are experiencing financial hardship, are uninsured or functionally
uninsured, are US residents, and received a prescription for a use indicated in the approved label of Dysport, as supported by information provided in the program application. Eligibility does not guarantee approval for participation in the program. The PAP provides Dysport product only, and does not cover the cost of previously purchased product or medical services.
Postmarketing reports indicate that the effects of Dysport and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity, but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses and in approved indications, cases of spread of effect have been reported at doses comparable to or lower than the maximum recommended total dose.
Dysport is contraindicated in patients with known hypersensitivity to any botulinum toxin products, cow’s milk protein, components in the formulation or infection at the injection site(s). Serious hypersensitivity reactions including anaphylaxis, serum sickness, urticaria, soft tissue edema, and dyspnea have been reported. If such a reaction occurs, discontinue Dysport and institute appropriate medical therapy immediately.
Lack of Interchangeability Between Botulinum Toxin Products
The potency Units of Dysport are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products, and, therefore, units of biological activity of Dysport cannot be compared to or converted into units of any other botulinum toxin products assessed with any other specific assay method.
Dysphagia and Breathing Difficulties
Treatment with Dysport and other botulinum toxin products can result in swallowing or breathing difficulties. Patients with pre-existing swallowing or breathing difficulties may be more susceptible to these complications. In most cases, this is a consequence of weakening of muscles in the area of injection that are involved in breathing or swallowing. When distant side effects occur, additional respiratory muscles may be involved. Deaths as a complication of severe dysphagia have been reported after treatment with botulinum toxin. Dysphagia may persist for several weeks, and require use of a feeding tube to maintain adequate nutrition and hydration. Aspiration may result from severe dysphagia and is a particular risk when treating patients in whom swallowing or respiratory function is already compromised. Patients treated with botulinum toxin may require immediate medical attention should they develop problems with swallowing, speech, or respiratory disorders. These reactions can occur within hours to weeks after injection with botulinum toxin.
Pre-existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junction disorders (e.g., myasthenia gravis or Lambert-Eaton syndrome) should be monitored particularly closely when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including severe dysphagia and respiratory compromise from typical doses of Dysport.
Human Albumin and Transmission of Viral Diseases
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases and variant Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk of transmission would also be considered extremely remote. No cases of transmission of viral diseases, CJD, or vCJD have ever been identified for licensed albumin or albumin contained in other licensed products.
Intradermal Immune Reaction
The possibility of an immune reaction when injected intradermally is unknown. The safety of Dysport for the treatment of hyperhidrosis has not been established. Dysport is approved only for intramuscular injection.
Adults with lower limb spasticity (≥5%): falls, muscular weakness, and pain in extremity and with upper limb spasticity (≥4%): muscular weakness.
Pediatric patients with lower limb spasticity (≥10%): nasopharyngitis, cough and pyrexia and with upper limb spasticity (≥10%): upper respiratory tract infection and pharyngitis.
Adults with cervical dystonia (≥5%): muscular weakness, dysphagia, dry mouth, injection site discomfort, fatigue, headache, musculoskeletal pain, dysphonia, injection site pain, and eye disorders.
Co-administration of Dysport and aminoglycosides or other agents interfering with neuromuscular transmission (e.g., curare-like agents), or muscle relaxants, should be observed closely because the effect of botulinum toxin may be potentiated. Use of anticholinergic drugs after administration of Dysport may potentiate systemic anticholinergic effects, such as blurred vision. The effect of administering different botulinum neurotoxins at the same time or within several months of each other is unknown. Excessive weakness may be exacerbated by another administration of botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin. Excessive weakness may also be exaggerated by administration of a muscle relaxant before or after administration of Dysport.
There are no adequate and well-controlled studies in pregnant women. Dysport should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Based on animal data, Dysport may cause fetal harm.
The safety and effectiveness of Dysport injected into proximal muscles of the lower limb for the treatment of spasticity in pediatric patients has not been established. Based on animal data Dysport may cause atrophy of injected and adjacent muscles; decreased bone growth, length, and mineral content; delayed sexual maturation; and decreased fertility.
In general, elderly patients should be observed to evaluate their tolerability of Dysport, due to the greater frequency of concomitant disease and other drug therapy. Subjects aged 65 years and over who were treated with Dysport for lower limb spasticity reported a greater percentage of fall and asthenia as compared to those younger (10% vs. 6% and 4% vs. 2%, respectively).
To report SUSPECTED ADVERSE REACTIONS or product complaints, contact Ipsen at 1-855-463-5127. You may also report SUSPECTED ADVERSE REACTIONS to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Dysport® (abobotulinumtoxinA) for injection is indicated for the treatment of:
Please see full Prescribing Information, including Boxed Warning and Medication Guide.